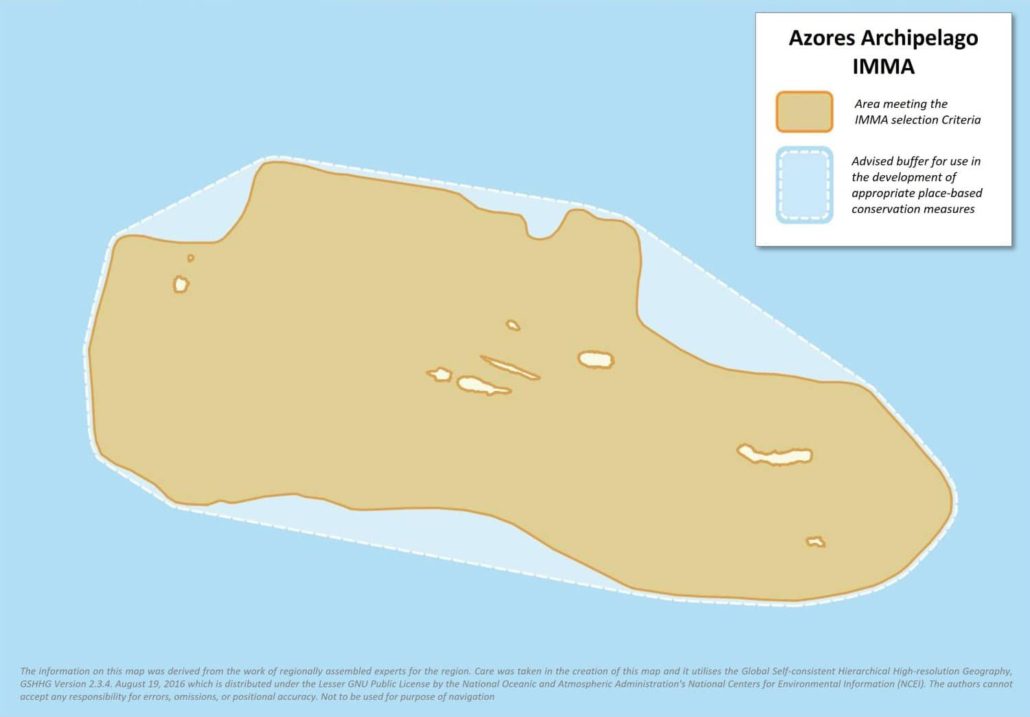
Azores Archipelago IMMA
Size in Square Kilometres
202,062 km2
Qualifying Species and Criteria
Sperm whale – Physeter macrocephalus
Criterion A; B (2); C (1,2)
Blue whale – Balaenoptera musculus
Criterion A; C (2)
Fin whale – Balaenoptera physalus
Criterion A; C (2)
Sei whale – Balaenoptera borealis
Criterion A; C (2)
Risso’s dolphin – Grampus griseus
Criterion B (1); D (1)
Sowerby’s beaked whale – Mesoplodon bidens
Criterion B (2)
Marine Mammal Diversity
Criterion D (2)
Grampus griseus, Physeter macrocephalus, Balaenoptera musculus, Balaenoptera borealis, Balaenoptera physalus, Mesoplodon bidens, Delphinus delphis, Stenella frontalis, Tursiops truncatus, Stenella coeruleoalba, Ziphius cavirostris, Mesoplodon densirostris, Hyperoodon ampullatus, Globicephala macrorhynchus, Pseudorca crassidens, Orcinus orca, Balaenoptera edeni, Balaenoptera acutorostrata, Megaptera novaeangliae
Download fact sheet
Summary
The remote archipelago of the Azores is characterised by dynamic oceanography and complex bathymetry, providing an exceptional habitat for cetaceans. It benefits from a sustainable development model based on artisanal fisheries with limited cetacean bycatch, and one of the largest MPA networks in the Atlantic covering coastal, oceanic and deep-sea habitats. The area hosts a small, resident population of Risso’s dolphins (Grampus griseus), and important aggregations of Vulnerable sperm whales (Physeter macrocephalus) and Sowerby’s beaked whales (Mesoplodon bidens). Additionally, it serves as foraging, calving and possibly mating ground for sperm whales, and as a mid-latitude foraging area for migratory Endangered blue (Balaenoptera musculus) and sei (B. borealis) whales, and Vulnerable fin whales (B. physalus). It hosts a high cetacean diversity with 19 species regularly sighted, including six baleen whale species, eight delphinid species, four beaked whale species, and sperm whales.
Description of Qualifying Criteria
Criterion A – Species or Population Vulnerability
Blue whales (Balaenoptera musculus) and sei whales (Balaenoptera borealis) are currently listed as Endangered on the IUCN Red List of Threatened Species (Cooke, 2018a, b), and fin whales (Balaenoptera physalus) (Cooke, 2018c) and sperm whales (Physeter macrocephalus) as Vulnerable (Taylor et al., 2019). The European regional Red List considers sperm whales as Vulnerable, while blue, sei and fin whales are considered Endangered (Cabral et al., 2005). The region is a well-known feeding and calving ground for sperm whales, and evidence suggests it serves as mid-latitude foraging habitat for fin, blue and sei whales during spring migration (Clarke, 1993; Prieto et al., 2014; Silva et al., 2013, 2014, 2019; González García et al., 2018; 2022; Pérez-Jorge et al., 2020). Additionally, these last three species have been acoustically detected around the archipelago in autumn and winter (Romagosa et al., 2020).
Criterion B: Distribution and Abundance
Sub-criterion B1: Small and Resident Populations
Risso’s dolphins (Grampus griseus) are listed as Least Concern globally (Kiszka and Braulik, 2018) and also Least Concern within European waters (Genov 2023). Sightings of Risso’s dolphins have been reported to occur throughout the entire Azorean archipelago (Silva et al., 2014; Tobeña et al., 2016) but there are indications of a resident population around one of the islands.
Out of 1,626 individuals identified south of Pico in 2004-2021, 223 were resighted more than 25 times, and 130 were resighted over 50 times, indicating strong site fidelity to the area (K.L. Hartman, unpublished data). This corroborates a previous study based on data collected over 35 consecutive months (May 2004 – November 2007) that showed that this population was resident year-round in the area (Hartman et al., 2015). Home range analysis of frequently sighted individuals revealed core habitat areas within 4-nautical miles south of Pico Island (Hartman et al., 2014, 2015). Within this area, females with neonates and nursing calves appear to use coastal waters more frequently, often less than 100 m from the shore, whereas sub-adult and adult groups (mixed sex or males only) occupy slightly more offshore waters (Hartman et al., 2015). During the nursing period, between June and August, sub-adults, pregnant females, and females with neonates and older calves often cluster together in nurseries, occupying restricted core areas (Hartman et al., 2014).
Sub-criterion B2: Aggregations
The global population of sperm whales in 2022 was estimated at 844,761 (SE = 171,206; CV = 0.209; 95% CI 481,901–1,153,459) individuals, which represents a 57% (95% CI 28.43–75.56) decline from the pre-whaling (reference year of 1711) population size (Whitehead & Shin, 2022). The same authors provide an estimate of 92,085 (CV=0.38) sperm whales for the North Atlantic in 1993, representing 12.5% of the global population of this species. Sperm whales are frequently sighted around all the islands of the Azores, as well as in more offshore waters (encounter rates of 0.44/100 km in habitats <20 km from the islands, and of 0.22/100 km in habitats <100 km from the islands) (Silva et al., 2014; Tobeña, 2022). Whales of both sexes and all age classes use the area year-round, but the majority (76%) of observations consist of social groups, and sighting rates are higher in late spring and summer (Silva et al., 2014). Sperm whale social groups are nomadic (Whitehead et al. 2008), and although a few social groups use the area nearly every year, none resides in the area permanently (Boys et al., 2019). There are no estimates of abundance of sperm whales for the entire Azores region. Capture-recapture models applied to photo-identification data collected during the summer (July-early September) 2009-2015 around only two islands (~8,500 km2, ~6% of the cIMMA), estimated that a total population of 1468 (95% CI: 1203–1791) adult and juvenile sperm whales used the area in those years as part of their summer range (Boys et al., 2019). However, not all whales from this super-population visited the area every summer, and annual abundance ranged between 275 (95% CI: 188–404) in 2014 and 367 (95% CI: 248–543) in 2012 (Boys et al., 2019). A ship-based line transect survey conducted in the summer of 2018 around the central group of islands of the Azores (32,804 km2) yielded an estimate of 212 sperm whales (95% CI: 134–334) (Freitas et al., 2019), although this estimate was not corrected for perception or availability bias, and therefore it is likely underestimated. The uncorrected average density of 0.006 whales/km2 is 1.5 to 3 times higher than the density estimated in the Northeast Atlantic (Rogan et al., 2017).
Beaked whales (Ziphiidae) are frequently sighted around the Azores islands as well as in more offshore waters (Silva et al., 2014, Tobeña et al., 2016). Although no population estimates are available for the whole archipelago, estimates of beaked whale density from the ship line-transect survey around the central islands of the Azores were considerably higher than those in neighbouring regions (archipelagos of Madeira and Canary Islands) (Freitas et al., 2019), suggesting that the Azores hosts important aggregations of multiple beaked whale species. This is especially the case for Sowerby’s beaked whale (Mesoplodon bidens), with an estimated abundance of 605 whales (CV=28%, 95%CI: 355-1,032) in the 32,804 km2 survey area (<24% of the cIMMA) (Freitas et al., 2019), giving an estimated density of 0.018 whales/km2. These estimates were not corrected for availability or perception bias and therefore are very likely underestimates.
C: Key Life Cycle Activities
Sub-criterion C1: Reproductive Areas
The IMMA is used as a calving, and possibly mating, ground by sperm whales (Clarke, 1981; Silva et al., 2014). About 76% of sperm whale sightings in the area are of social groups, 8% of adult males, observed singly or in aggregations, and the remaining 16% of adult males interacting with social units (Silva et al., 2014). Calves are frequently sighted (38% of the sightings include at least one calf) throughout the year, although the proportion of sightings with calves is higher in summer (Silva et al., 2014). Newborns (<5 m, with foetal folds) are also frequently observed, with a clear peak in August (Silva et al., 2014), agreeing with historical whaling data that showed an increasing rate of large foetuses and lactating female sperm whales in summer, supporting their birth during July and August (Clarke, 1956). Parturition and sperm whale placentas have been reported occasionally by whale-watch operators.
Sub-criterion C2: Feeding Areas
Analysis of stomach contents from sperm whales hunted in the Azores showed that whales foraged in the area (Clarke, 1956; Clarke, 1983). Consistent with this, most whales encountered in the Azores are foraging (Silva et al., 2014) and individual whales have been documented to spend 75-80% of their time foraging (Gordon and Steiner, 1992). Diving profiles of sperm whales instrumented with Dtags showed that whales spent about 76% of the time in foraging dives to the lower mesopelagic layer (bottom phase diving depth of 750 m), producing an average of 0.53 (±0.19) buzzes per minute, suggesting high rates of prey capture attempts (Oliveira et al., 2022). The predicted relative density of sperm whales in the region is higher over deep waters (1,000-2,200 m depth) and is significantly associated with micronekton production in mesopelagic layers, which probably attracts squid species that sperm whales feed on (Tobeña, 2022).
Blue, fin and sei whales are observed every year in the Azores region, mostly between March and July, with occasional sightings in autumn and winter (Silva et al., 2014; Visser et al., 2011; González García et al., 2018, 2022). These species use the area as mid-latitude foraging habitat during spring migration (Silva et al., 2013). Satellite tracking data showed that blue and fin whales suspended their spring migration and remained within the wider Azores region for periods ranging from from days to a few months, spending a substantial proportion of their time in area-restricted search behaviour, assumed to represent foraging (Silva et al., 2013). This is supported by recent studies of the diving behaviour and activity budgets of fin whales (Fonseca et al., 2022). Conversely, tracking data and at-sea observations suggested that sei whales had shorter residency times in the area and rarely engaged in foraging activity (Prieto et al., 2014). However, in recent years, sei whales have been observed in the area for months foraging on small fish (especially snipe fish Macroramphosus sp.). Modelling of tracking data showed that movement patterns of blue, fin and sei whales within the Azores and at higher latitudes were closely linked to prey biomass and production within the epipelagic layer, consistent with their known diets (Pérez-Jorge et al., 2020).
Criterion D: Special Attributes
Sub-criterion D1: Distinctiveness
The existence of genetically distinct populations of Risso’s dolphins in the northern North Atlantic (United Kingdom, UK) and Mediterranean waters has been reported by Gaspari et al. (2007). More recently, analysis of mitochondrial DNA showed that, despite a lack of complete lineage sorting, Risso’s dolphins inhabiting the waters of the Azores were genetically differentiated from those sampled in the UK, Mediterranean and Pacific Ocean (Chen et al., 2018). In fact, no haplotype was shared between the Azores (15 unique haplotypes, 35 samples) and the UK (3 haplotypes, 18 samples) or Pacific Ocean (55 haplotypes, 136 samples), and only one haplotype was shared with the Mediterranean (13 haplotypes, 24 samples). In addition, morphological differences have been reported for Risso’s dolphins from different regions (e.g., Japan and Taiwan, Chen et al., 2011; Yu et al., 2019; Faroe Islands, Bloch et al., 2012; South Africa, Plön et al., 2020; Azores, Hartman et al., 2016, 2023), reflecting restricted gene flow and genetic divergence between populations, and/or adaptation to local ecological conditions through phenotypic plasticity.
Risso’s dolphins in the Azores maintain a sexually stratified community with males forming stable clusters, whereas females tend to associate in temporally stable units when calving and nursing (Hartman et al., 2008). In addition, the mating system of this species reveals complex patterns and layered intra-group male-male collaborations (Hartman et al., 2020). Male Risso’s dolphins have been frequently observed to conduct consortships, which is considered to be a driver of the rare existence of non-kin but stable associated multi-male groups in the research area (Hartman et al., 2023).
Sub-criterion D2: Diversity
The Azores hosts some of the highest cetacean biodiversity in the world, with 19 species of toothed and baleen whales occurring regularly in the region (Silva et al., 2014; Azevedo and Barreiros, 2019; Azevedo et al., 2023). This includes a mix of resident common bottlenose (Tursiops truncatus) and Risso’s dolphin populations, species that are present year-round (e.g. sperm whales, common (Delphinus delphis) and striped dolphins (Stenella coeruleoalba), Cuvier’s (Ziphius cavirostris), Sowerby’s and Blainville’s (Mesoplodon densirostris) beaked whales, and seasonal visitors, such as blue, fin and sei whales, Bryde’s whales (Balaenoptera edeni), Atlantic spotted dolphins (Stenella frontalis) and northern bottlenose whales (Hyperoodon ampullatus). There are also regular visitors with no clear seasonality, such as short-finned pilot whales (Globicephala macrorhynchus), false killer whales (Pseudorca crassidens), common minke whales (Balaenoptera acutorostrata), humpback whales (Megaptera novaeangliae), and killer whales (Orcinus orca) (Silva et al. 2014).
Sightings of dwarf (Kogia sima) and pygmy sperm whales (Kogia breviceps), Gervais’ (Mesoplodon europaeus) and True’s beaked whales (Mesoplodon mirus) are rare but strandings suggest that these taxa species are present year-round and possibly in higher numbers than revealed by sighting information (Silva et al. 2014). In addition, long-finned pilot whales (Globicephala melas) and rough-toothed dolphins (Steno bredanensis) are occasionally recorded in the area.
Supporting Information
Afonso, P., Fontes, J., Giacomello, E., Magalhães, M., Martins, H.R., Morato, T., Neves, V., Prieto, R., Santos, R.S., Silva, M.A. and Vandeperre, F. (2020). ‘The Azores: a mid-Atlantic hotspot for marine megafauna research and conservation.’ Perspective article, Frontiers in Marine Science – Deep-Sea Environments and Ecology. DOI: 10.3389/fmars.2019.00826.
Azevedo, J. and Barreiros, J. P. (2019). ‘Azores cetaceans updated checklist’. Version 1.1. Universidade dos Açores. Checklist dataset. DOI: 10.15468/dmrkx9 accessed via GBIF.org on 2023-04-08.
Azevedo, J.M.N., Fernández, M. and González García, L. (2023). ‘MONICET: long-term cetacean monitoring in the Azores based on whale watching observations (2009-2020)’. DOI: 10.14284/599 accessed on 2023-05-01.
Boys, R. M., Oliveira, C., Pérez-Jorge, S, Prieto, R., Steiner, L. and Silva, M. A. (2019). ‘Multi-state open robust design applied to opportunistic data reveal dynamics of wide-ranging taxa: the sperm whale case.’ Ecosphere, 10(3): e02610. DOI: 10.1002/ecs2.2610.
Bloch, D., Desportes, G., Harvey, P., Lockyer, C. and Mikkelsen, B. (2012). ‘Life history of Risso’s dolphin (Grampus griseus) (G. Cuvier, 1812) in the Faroe Islands.’ Aquatic Mammals, 38: 250–266
Cabral, M.J. (coord), Almeida, J., Almeida, P.R., Delliger, T., Ferrand de Almeida, N., Oliveira, M.E., Palmeirim, J.M., Queirós, A.I., Rogado, L. and Santos-Reis, M. (eds.) (2005). Livro Vermelho dos Vertebrados de Portugal. Instituto da Conservação da Natureza. Lisboa. 659p.
Chen, I., A. Watson, and L.-S. Chou. (2011). ‘Insights from life history traits of Risso’s dolphins (Grampus griseus) in Taiwanese waters: Shorter body length characterizes northwest Pacific population.’ Marine Mammal Science, 27, E43–E64
Chen, I., Nishida, S., Chou, L.S., Taijma, Y., Tang, W.C., Isobe, T., Yamada, T.K., Hartman, K.L. and Hoelzel, A.R. (2018). ’Concordance between genetic diversity and marine biogeography in a highly mobile marine mammal, the Risso’s dolphin.’ Journal of Biogeography, 45(9) 2092-2103. DOI: 10.1111/jbi.13360.
Clarke, M.R., Martins, H.R. and Pasccoe, P. (1993). ‘The diet of sperm whales (Physeter macrocephalus, Linnaeus 1758) off the Azores.’ Phil Trans R Soc Lond B, 339: 67-82.
Clarke, R. (1956). ‘Sperm Whales of the Azores’. Discovery Reports, vol. XXVIII, pp. 237-298, Plates I, II.
Clarke, R. (1981). ‘Whales and dolphins of the Azores and their exploitation.’ Reports of the International Whaling Commission, 31:607-615.
Cooke, J.G. (2018a). ‘Balaenoptera musculus (errata version published in 2019). The IUCN Red List of Threatened Species 2018: e.T2477A156923585.’ https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T2477A156923585.en. Accessed on 05 April 2023.
Cooke, J.G. (2018b). ‘Balaenoptera borealis. The IUCN Red List of Threatened Species 2018: e.T2475A130482064.’ https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T2475A130482064.en. Accessed on 05 April 2023.
Cooke, J.G. (2018c). ‘Balaenoptera physalus. The IUCN Red List of Threatened Species 2018: e.T2478A50349982.’ https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T2478A50349982.en. Accessed on 05 April 2023.
Fonseca, C.T., Pérez-Jorge, S., Prieto, R., Oliveira, C., Tobeña, M., Scheffer, A. And Silva, M.A. (2022). ‘Dive behavior and activity patterns of fin whales in a migratory habitat.’ Frontiers in Marine Science, 2022; 9:875731. DOI: 10.3389/fmars.2022.875731.
Freitas, L., Cañadas, A., Servidio, A., Pérez-Gil, A., Pérez-Gil, E., Varo-Cruz, N., Silva, M., Vandeperre, F. and Esteban, R. (2019). ‘A-MB-TR2 – Technical Report – Sub-programmes Abundance of Oceanic Cetaceans (MM) and Loggerhead Census (MT)- Oceanic. MISTIC SEAS II – Applying a sub-regional coherent and coordinated approach to the monitoring and assessment of marine biodiversity in Macaronesia for the second cycle of the MSFD (11.0661/2017/750679/SUB/ENV.C2).’ DOI:10.13140/RG.2.2.13417.11369.
Gaspari, S., Airoldi, S. and Hoelzel, A.R. (2007). ‘Risso’s dolphins (Grampus griseus) in UK waters are differentiated from a population in the Mediterranean Sea and genetically less diverse.’ Conserv Genet 8:727–732. https://doi.org/10.1007/s10592-006-9205-y.
Genov, T. 2023. Grampus griseus (Europe assessment). The IUCN Red List of Threatened Species 2023: e.T9461A218567526. Accessed on 29 April 2024.
González García, L. (2019). ‘Cetacean distribution in São Miguel (Azores): influence of environmental variables at different spatial and temporal scales’. PhD thesis, University of Vigo. http://hdl.handle.net/11093/1218.
González García, L., Pierce, G. J., Autret, E. and Torres-Palenzuela, J. M. (2018). ‘Multi-scale habitat preference analyses for Azorean blue whales’. PloS ONE 13(9), e0201786. DOI: 10.1371/journal.pone.0201786.
González García, L., Pierce, G. J., Autret, E. and Torres-Palenzuela, J. M. (2022). ‘Alongside but separate: Sympatric baleen whales choose different habitat conditions in São Miguel, Azores’. Deep Sea Research Part I: Oceanographic Research Papers 184, 103766. DOI: 10.1016/j.dsr.2022.103766.
Gordon, J. and Steiner, L. (1992). ‘Ventilation and dive patterns in sperm whales, Physeter macrocephalus, in the Azores’. Rep. Int. Whal. Comm. 42: 561–565.
Hartman, K. L., Fernandez, M., Wittich, A. and Azevedo, J.M.N. (2015). ‘Sex differences in residency patterns of Risso’s dolphins (Grampus griseus) in the Azores: causes and management implications.’ Marine Mammal Science, 31(3): 1153–1167.
Hartman, K., van der Harst, P. and Vilela, R. (2020). ‘Continuous focal group follows operated by a drone enable analysis of the relation between sociality and position in a group of male Risso’s dolphins (Grampus griseus).’ Frontiers in Marine Science, 7, 283. DOI: 10.3389/fmars.2020.00283.
Hartman, K., van der Harst, P. And Vilela, R. (2023). ‘Sex and sexual strategies in deep diving Risso’s dolphins.’ In: Sex in cetaceans. Edited by: B. Wursig and D. Orbach, published by Springer Nature, Switzerland.
Hartman, K.L., Fernandez, M. and Azevedo, J.M.N. (2014). ‘Spatial segregation of calving and nursing Risso’s dolphins (Grampus griseus) in the Azores, and its conservation implications.’ Marine Biology, 161(6): 1419-1428.
Hartman, K.L., Visser, F. and Hendriks, A.J.E. (2008). ‘Social structure of Risso’s dolphins (Grampus griseus) at the Azores: a stratified community based on highly associated social units.’ Canadian Journal of Zoology, 86(4): 294-306. DOI: 10.1139/Z07-138.
Hartman, K. L., Wittich, A., Cai, J. J., Van Der Meulen, F. H., & Azevedo, J. M. (2016). ‘Estimating the age of Risso’s dolphins (Grampus griseus) based on skin appearance.’ Journal of Mammalogy, 97(2), 490-502.
Kiszka, J. and Braulik, G. (2018). ‘Grampus griseus. The IUCN Red List of Threatened Species 2018: e.T9461A50356660.’ https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T9461A50356660.en. Accessed on 11 April 2023.
Oliveira, C., Pérez-Jorge, S., Prieto, R., Cascão, I., Wensveen, P.J. and Silva, M.A. (2022). ‘Exposure to whale watching vessels affects dive ascents and resting behavior in sperm whales.’ Frontiers in Marine Science. 9:914397. DOI: 10.3389/fmars.2022.914397.
Pérez-Jorge, S., Tobeña, M., Prieto, R., Vandeperre, F., Calmettes, B., Lehodey, P. and Silva, M.A. (2020). ‘Environmental drivers of large-scale movements of baleen whales in the mid-North Atlantic Ocean.’ Diversity and Distributions. DOI:10.1111/ddi.13038.
Plön, S., Heyns‐Veale, E. R., Smale, M. J., & Froneman, P. W. (2020). ‘Life history parameters and diet of Risso’s dolphins, Grampus griseus, from southeastern South Africa.’ Marine Mammal Science, 36(3), 786-801.
Prieto, R., Silva, M. A., Waring, G. T. & Gonçalves, J. M. A. (2014). ‘Sei whale movements and behaviour in the North Atlantic inferred from satellite telemetry’. Endangered Species Research 26, 103–113. DOI: https://doi.org/10.3354/esr00630.
Prieto, R., Tobeña, M. and Silva, M. A. (2017). ‘Habitat preferences of baleen whales in a mid-latitude habitat.’ Deep Sea Research Part II: Topical Studies in Oceanography, 141, 155-167. DOI: 10.1016/j.dsr2.2016.07.015.
Rogan, E., Cañadas, A., Macleod, K., Santos, M. B., Mikkelsen, B., Uriarte, A., et al. (2017). ‘Distribution, abundance and habitat use of deep diving cetaceans in the North-East Atlantic.’ Deep Sea Research Part II: Topical Studies in Oceanography, 141, 8-19. DOI:10.1016/j.dsr2.2017.03.015.
Romagosa, M., Baumgartner, M., Cascão, I., Lammers, M-O., Marques, T.A., Santos, R.S. and Silva, M.A. (2020). ‘Baleen whale acoustic presence and behaviour at a Mid-Atlantic migratory habitat, the Azores Archipelago.’ Scientific Reports 10, 4766. DOI: 10.1038/s41598-020-61849-8.
Silva, M. A., Prieto, R., Cascão, I., Seabra, M.I., Machete, M., Baumgartner, M.F. and Santos, R.S. (2014). ‘Spatial and temporal distribution of cetaceans in the mid-Atlantic waters around the Azores.’ Marine Biology Research 10:123-137.
Silva, M. A., Prieto, R., Jonsen, I., Baumgartner, M.F. and Santos, R.S. (2013). ‘North Atlantic blue and fin whales suspend their spring migration to forage in middle latitudes: building up energy reserves for the journey?’ PLoS ONE 8:e76507.
Silva, M.A., Borrell, A., Prieto, R., Gauffier, P., Bérubé, M., Palsbøl, P.J. and Colaço, A. (2019). ‘Stable isotopes reveal winter feeding in different habitats in blue, fin and sei whales migrating through the Azores.’ R. Soc. Open Sci. 6: 181800. http://dx.doi.org/10.1098/rsos.1818009.
Taylor, B.L., Baird, R., Barlow, J., Dawson, S.M., Ford, J., Mead, J.G., Notarbartolo di Sciara, G., Wade, P. and Pitman, R.L. (2019). ‘Physeter macrocephalus (amended version of 2008 assessment). The IUCN Red List of Threatened Species 2019: e.T41755A160983555.’ DOI: 10.2305/IUCN.UK.2008.RLTS.T41755A160983555.en. Accessed on 05 April 2023.
Species account by IUCN SSC Cetacean Specialist Group; regional assessment by European Mammal Assessment team. (2007). ‘Grampus griseus (Europe assessment). The IUCN Red List of Threatened Species 2007: e.T9461A12989112.’ Accessed on 31 October 2023.
Tobeña, M. (2022). ‘Near real-time distribution modelling of cetacean distribution off the Azores.’ PhD Thesis, University of the Azores.
Tobeña, M., Prieto, R., Machete, M. and Silva, M.A. (2016). ‘Modeling the Potential Distribution and Richness of Cetaceans in the Azores from Fisheries Observer Program Data.’ Front. Mar. Sci. 3:202. DOI: 10.3389/fmars.2016.00202.
Visser, F., Hartman, K.L., Pierce, G.J., and Valavanis, V.D. (2011). ‘Timing of migratory baleen whales at the Azores in relation to the North Atlantic spring bloom.’ Marine Ecology Progress Series, 440, 267–279. DOI: 10.3354/meps0 9349.
Whitehead, H. and Shin, M. (2022). ‘Current global population size, post-whaling trend and historical trajectory of sperm whales.’ Scientific Reports, 12(1), 19468. DOI: 10.1038/s41598-022-24107-7.
Yu, H.-Y., Chen, I., Li, W.-T., Chou, L.-S. (2019). ‘Ecological and Biological Characteristics for the Risso’s Dolphins (Grampus griseus) Off Taiwan, with Conservation Evaluations on Potential Anthropogenic Threats.’ Mammal Study 44, 1. https://doi.org/10.3106/ms2018-0038
Downloads
Download the full account of the Azores Archipelago IMMA using the Fact Sheet button below:
To make a request to download the GIS Layer (shapefile) for the Azores Archipelago IMMA please complete the following Contact Form: