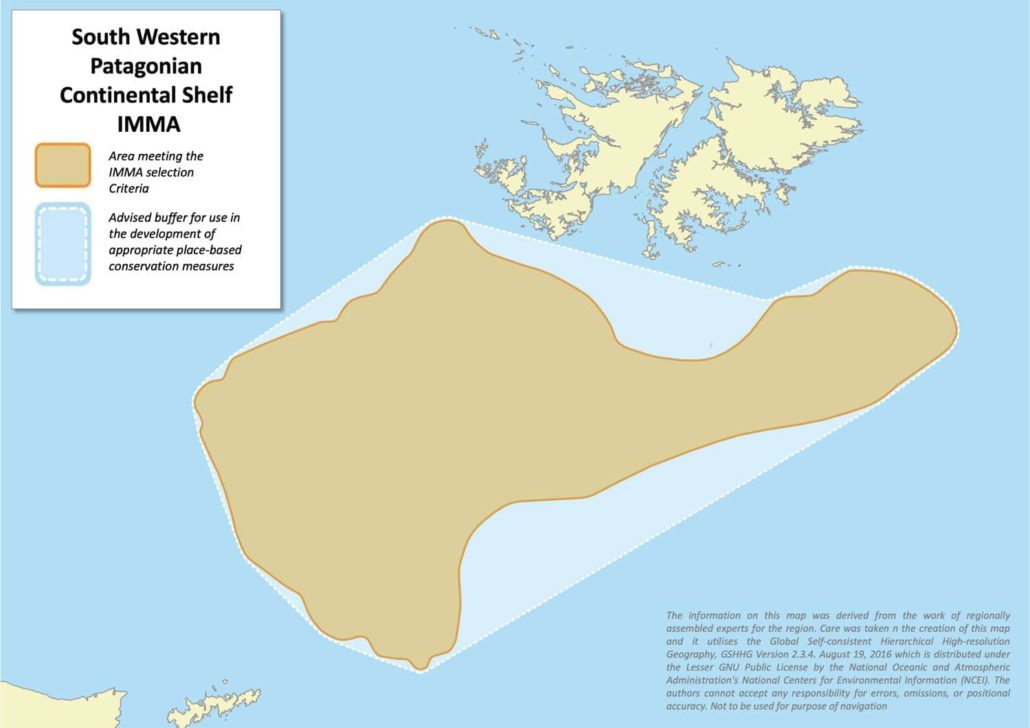
Size in Square Kilometres
61999
Qualifying Species and Criteria
Southern elephant seal – Mirounga leonina
Criterion C (2); D (1)
Criterion D (2) – Marine Mammal Diversity
Other Marine Mammal Species Documented
Summary
Sea Lion Island is the main breeding colony of southern elephant seals (Mirounga leonina) in the Falkland Islands (Malvinas)*, and is the only one regularly monitored, and the only one showing a proven increase in numbers over time. Although Sea Lion Island breeding females show a variety of feeding strategies, most of them forage in localized areas of a small part of the Southern Patagonian Shelf, located between the Islands and the Burdwood Bank, relatively close to the breeding colony and in relatively shallow waters. This area is very important for a crucial part of the life cycle of the breeding females, which are the key demographic component for the future of the species in the Islands. Moreover, their feeding strategy is different from the long loops in deep waters at the edge or outside of the continental shelf adopted by females from other populations of the South Georgia stock. At least 18 cetacean species have ranges overlapping with the IMMA but there is as yet little information on their occurrence.
*Since 1965 the nomenclature used by the United Nations for statistical processing is Falkland Islands (Malvinas), which acknowledges the dispute that exists concerning the sovereignty of the Islands.
Description of Qualifying Criteria
Criterion A – Species or Population Vulnerability
Criterion B – Distribution and Abundance
Sub-criterion B1 – Small and Resident Populations
Sub-criterion B2 – Aggregations
Criterion C: Key Life Cycle Activities
Sub-criterion C1 – Reproductive Areas
Sub-criterion C2: Feeding Areas
The formerly very large southern elephant seal (Mirounga leonina) population of the Islands was exterminated during the commercial sealing operations of the 19th century (Strange 1972). The population showed little recovery, and currently comprises only small colonies, of uncertain status and not regularly monitored. The only exception to this is Sea Lion Island, which is not only the largest colony (730 breeding females, 2022 estimate, Elephant Seal Research Group, ESRG, unpublished data), but it is also the only one with a documented increasing trend in size (2.1% per year, 2003-2022, ESRG unpublished data, see also IMMA “Sea Lion Islands Group”). Therefore, the most important demographic class of that population, the breeding females, play a crucial role for the maintenance of the breeding presence of the species in the Islands. The majority of Sea Lion Island breeding females use the IMMA as their main feeding ground during the post-breeding migration at sea. Therefore, this area is not only very important for the conservation of the localized population of Sea Lion Island, but it is important for the conservation of the Islands biodiversity at large because it is the main feeding area of the main component of the Islands southern elephant seals.
Genetic differentiation of the populations of the South Georgia stock is low, with the only notable exception of the Valdes Peninsula (Fabiani et al. 2003). On the other hand, Sea Lion Island is demographically isolated (Galimberti and Boitani 1999), and breeding females show strong philopatry and breeding site fidelity (Fabiani et al. 2006). This is confirmed by a long-term tag-resight study (1995-2023, 28 fully tagged pup cohorts, ~16000 individuals; ESRG, unpublished data), which shows that: 1) there is no immigration of breeding females; 2) skipping of breeding, temporary emigration to other breeding colonies, and permanent emigration of females is very rare; 3) there is occasional immigration of males breeders (see also Fabiani et al. 2003) but it is exceedingly rare; 4) there is abundant haul out of Southern Elephant Seals from all other populations of the South Georgia stock but only during the moulting season and during the winter haul out.
Sub-criterion C3: Migration Routes
C3a – Whale Seasonal Migratory Route
C3b – Migration / Movement Area
Criterion D – Special Attributes
Sub-criterion D1 – Distinctiveness
Post-breeding southern elephant seal females usually carry out long migration and feeding loops over deep waters, very far from their breeding colonies (South Georgia: McConnell and Fedak 1996; Valdes Peninsula: Campagna et al. 1995). Sea Lion Island females show a variety of feeding strategies; some females adopt a feeding strategy similar to the ones mentioned above for other populations, moving along long loops either north of the Islands (13.0% of 23 females), south towards the Antarctic Peninsula (1 female) or going to the Pacific side of South America, in very deep waters in front of the coast of Chile (17.4 %; track maps in Galimberti and Sanvito2012). This variability and individuality of movements at sea are mirrored in the variability of feeding niche and diet as shown by stable isotopes analysis (Rita et al. 2017).
Notwithstanding this, the majority of the Sea Lion Island breeding elephant seal females (65.3%) show a specific foraging strategy based on a fast migration to a localized feeding area, followed by a rapid return to Sea Lion Island or nearby islands for the moult. This feeding area is located between the Islands and the Burdwood Bank, close to the breeding colony and in relatively shallow waters (maximum water depth of foraging fixes = -295 to -774) (Galimberti and Sanvito 2012).
The mean maximum distance from the breeding colony of the 13 females feeding in this area was 249 km (122-350), while it was 714 km (613-833) for three females that foraged north of the Islands, and 1386 km (1147-1691) for five females that foraged over long loops towards the Antarctic Peninsula or the Pacific Ocean. The mean maximum distance from the breeding colony reached during the post-breeding migration was 1031 km at Marion Island (607-1460, n = 9 females; Jonker and Bester 1998, Table 1) and 1022 km at Valdes Peninsula (813-1223, n = 6 females; Campagna et al. 1998, Table 2). Very long loops (maximum distance from breeding colony up to 3000 km) were also shown by South Georgia females (McConnell and Fedak 1996; see Appendix).
Hence, most Sea Lion Island elephant seal females show a rather unique feeding strategy, very different from the main foraging strategy of other populations of the South Georgia population. Overall, the strategy is apparently very successful, because the population is increasing and shows very little pre-weaning pup mortality (1-2% with little variation among cohorts; Galimberti and Boitani 1999), and the average weaning weight is on the high side of the species range (female weanling = 135 kg, male weanlings = 137kg, Galimberti and Boitani 1999) when compared to other populations (Burton et al. 1997).
Sub-criterion D2 – Diversity
Supporting Information
Agnew, D. J. (2002). Critical aspects of the Falkland Islands pelagic ecosystem: distribution, spawning and migration of pelagic animals in relation to oil exploration. Aquatic Conservation: Marine And Freshwater Ecosystems12: 39-50.
Arkipkin, A., P. Brickle and V. Laptikhovsky (2013). Links between marine fauna and oceanic fronts on the Patagonian Shelf and Slope. Arquipelago. Life and Marine Sciences 30: 19-37.
Baylis, A. M. M., M. Tierney, R. A. Orben, V. Warwick-Evans, E. Wakefield, W. J. Grecian, P. Trathan, R. Reisinger, N. Ratcliffe, J. Croxall, L. Campioni, P. Catry, S. Crofts, P. D. Boersma, F. Galimberti, J. P. Granadeiro, J. Handley, S. Hayes, A. Hedd, J. F. Masello, W. A. Montevecchi, K. Pütz, P. Quillfeldt, G. A. Rebstock, S. Sanvito, I. J. Staniland and P. Brickle (2019). Important At-Sea Areas of Colonial Breeding Marine Predators on the Southern Patagonian Shelf. Scientific Reports 9(1): 8517, doi: 10.1038/s41598-019-44695-1.
Burton, H. R., T. Arnbom, I. L. Boyd, M. N. Bester, D. Vergani and I. Wilkinson (1997). Significant differences in weaning mass of southern elephant seals from five sub-Antarctic islands in relation to population declines. Antarctic communities: species, structure and survival. B. Battaglia, J. Valencia and D. W. H. Walton. Cambridge, U.K., Cambridge University Press: 335-338.
Campagna, C., B. J. Le Boeuf, S. B. Blackwell, D. E. Crocker and F. Quintana (1995). Diving behaviour and foraging location of female southern elephant seals from Patagonia. Journal of Zoology236(1): 55-71.
Campagna, C., F. Quintana, B. J. Le Boeuf, S. Blackwell and D. E. Crocker (1998). Diving behaviour and foraging ecology of female southern elephant seals from Patagonia. Aquatic Mammals 24(1): 1-11.
Fabiani, A., A. R. Hoelzel, F. Galimberti and M. M. C. Meulbert (2003). Long-range paternal gene flow in the southern elephant seal. Science 299(5607): 676, doi: 10.1126/science.299.5607.676.
Fabiani, A., F. Galimberti, S. Sanvito and A. R. Hoelzel (2006). Relatedness and site fidelity at the southern elephant seal, Mirounga leonina, breeding colony in the Falkland Islands. Animal Behaviour 72(3): 617-626, doi: 10.1016/j.anbehav.2005.11.024.
Galimberti, F. and L. Boitani (1999). Demography and breeding biology of a small, localized population of southern elephant seals (Mirounga leonina). Marine Mammal Science 15(1): 159-178, doi: 10.1111/j.1748-7692.1999.tb00787.x
Galimberti, F. and S. Sanvito(2012). Tracking at sea the elephant seals of Sea Lion Island. ESRG Technical reports, Elephant Seal Research Group, Sea Lion Island, Falkland Islands. Available online from www.eleseal.org.
González, A., P. Trathan, C. Yau and P. Rodhouse (1997). Interactions between oceanography, ecology and fishery biology of the ommastrephid squid Martialia hyadesi in the South Atlantic. Marine Ecology Progress Series 152: 205-215, doi: 10.3354/meps152205.
Guerrero, R. A., A. G. Baldoni and H. R. Benavides (1999). Oceanographic conditions at the southern end of the argentine continental slope. INIDEP documentocientífico. Mar del Plata, Instituto Nacional de Investigación y Desarrollo Pesquero: 7-22.
Handley, J. M., E. Harte, A. Stanworth, S. Poncet, P. Catry, S. Cleminson, S. Crofts and M. Dias (2023). Progressing delineations of key biodiversity areas for seabirds, and their application to management of coastal seas. Diversity and Distributions 29(1): 123-142, doi: https://doi.org/10.1111/ddi.13651.
Jonker, F. C. and M. N. Bester (1998). Seasonal movements and foraging areas of adult southern female elephant seals, Mirounga leonina, from Marion Island. Antarctic Science 10(1): 21-30, doi: 10.1017/S0954102098000042.
Ludwig, W. J. (1983). Geological framework of the Falkland Plateau. Initial Report of the Deep Sea Drilling Project. W. J. Ludwig and V. A. Krashenninikov. 71: 281-293.
Marschoff, E., J. Calcagno, B. P. Ferreira, F. Lucena-Frédou, M. Muelbert, A. Perez, A. R. Rey, L. Schejter and A. Turra (2017). South Atlantic Ocean. The First Global Integrated Marine Assessment: World Ocean Assessment I. United_Nations. Cambridge, Cambridge University Press: 595-614.
McConnell, B. J. and M. A. Fedak (1996). Movements of southern elephant seals. Canadian Journal of Zoology 74(8): 1485-1496, doi: 10.1139/z96-163.
Miloslavich, P., E. Klein, J. M. Díaz, C. E. Hernández, G. Bigatti, L. Campos, F. Artigas, J. Castillo, P. E. Penchaszadeh, P. E. Neill, A. Carranza, M. V. Retana, J. M. Díaz de Astarloa, M. Lewis, P. Yorio, M. L. Piriz, D. Rodríguez, Y. Yoneshigue-Valentin, L. Gamboa and A. Martín (2011). Marine Biodiversity in the Atlantic and Pacific Coasts of South America: Knowledge and Gaps. PLOS ONE 6(1): e14631, doi: 10.1371/journal.pone.0014631.
Piola, A. R., E. D. Palma, A. A. Bianchi, B. M. Castro, M. Dottori, R. A. Guerrero, M. Marrari, R. P. Matano, O. O. Möller and M. Saraceno (2018). Physical Oceanography of the SW Atlantic Shelf: A Review. Plankton Ecology of the Southwestern Atlantic: From the Subtropical to the Subantarctic Realm. M. S. Hoffmeyer, M. E. Sabatini, F. P. Brandini, D. L. Calliari and N. H. Santinelli. Cham, Springer International Publishing: 37-56.
Rita, D., M. Drago, F. Galimberti and L. Cardona (2017). Temporal consistency of individual trophic specialization in southern elephant seals Mirounga leonina. Marine Ecology Progress Series585: 229-242, doi: 10.3354/meps12411.
Rodhouse, P. G., C. Symon and E. M. C. Hatfield (1992). Early life cycle of cephalopods in relation to the major oceanographic features of the Southwest Atlantic Ocean. Marine ecology progress series 89(2-3): 183-195, doi: 10.3354/meps089183.
Schejter, L., C. Rimondino, I. Chiesa, J. M. Díaz de Astarloa, B. Doti, R. Elías, M. Escolar, G. Genzano, J. López-Gappa, M. Tatián, D. G. Zelaya, J. Cristobo, C. D. Perez, R. T. Cordeiro and C. S. Bremec (2016). Namuncurá Marine Protected Area: an oceanic hot spot of benthic biodiversity at Burdwood Bank, Argentina. Polar Biology 39(12): 2373-2386, doi: 10.1007/s00300-016-1913-2.
Strange, I. (1972). Sealing industries of the Falkland Islands. The Falkland Islands Journal: 13-21.
Urien, C. M. and J. J. Zambrano (1973). The Geology of the Basins of the Argentine Continental Margin and Malvinas Plateau. The South Atlantic. A. E. M. Nairn and F. G. Stehli. Boston, MA, Springer US: 135-169.
Downloads
Download the full account of the South Western Patagonian Continental Shelf IMMA using the Brochure button below:
To make a request to download the GIS Layer (geopackage and/or geojson) for the South Western Patagonian Continental Shelf IMMA please complete the following Contact Form: